Frodo: a non-invasive, unobtrusive, and continuous bladder monitoring in real time
Frodo is not only a brave hobbit of the Shire, but a smart urinary bladder volume monitor that can bring down the use of catheter insertions and hence reduce the incidence of urinary tract infections and other associated complications. It has been conceived, designed and developed due to a successful collaboration among the D-ITET PBL Center (Dr. Michele Magno), D-ITET IIS (Prof. Luca Benini) and D-HEST (Prof. Simone Schürle).
Frodo’s quest
Catheter acquired urinary tract infection (CAUTI) is one of the most common healthcare acquired infections leading to prolonged stay and worse patient outcomes. Chronic use of indwelling or intermittent catheters to monitor urine output leads to higher risk of infection and increased mortality. Urine output collected from catheters is manually recorded by health workers intermittently, increasing not only the risk of human error but also adding to the workload of the health worker in limited resource scenario. Moreover, catheter insertion is uncomfortable and may cause lesions that further aggravate the problem. There are several risk factors for CAUTIs, such as prolonging the duration of the catheter, female sex, older age, diabetes mellitus, the absence of systemic antibiotics, catheter insertion outside the operating room, and a breach in the closed system of catheter drainage.
How does Frodo master his tasks?
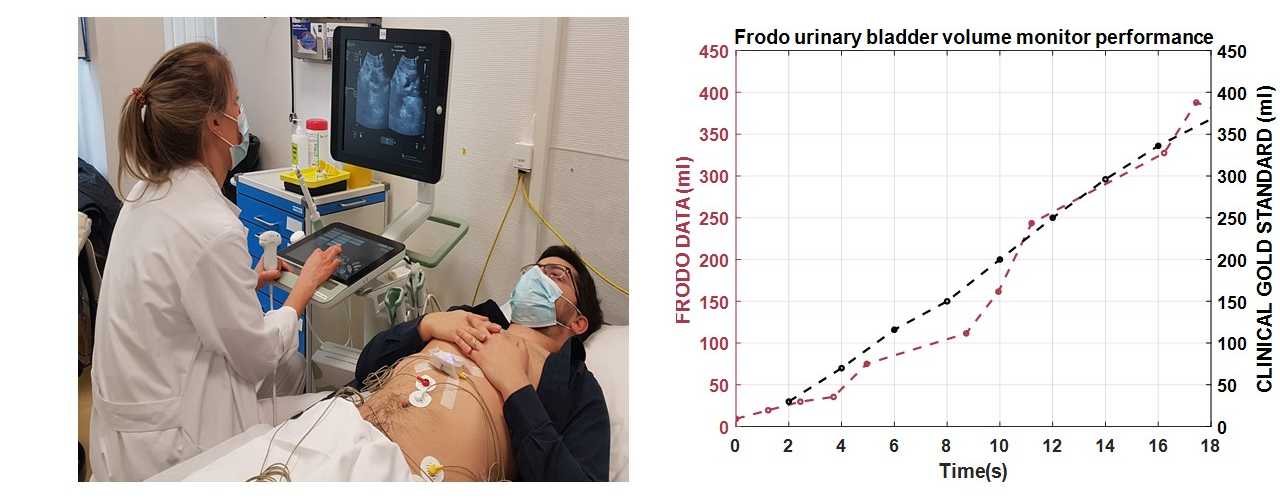
Frodo has been designed with innovative bio-impedance sensors distributed in an innovative way on the human body. A novel algorithm based on artificial intelligence studied during Kanika’s PhD filters the data and accurately extracts the level of urine in the bladder even in presence of artifacts and body movements.
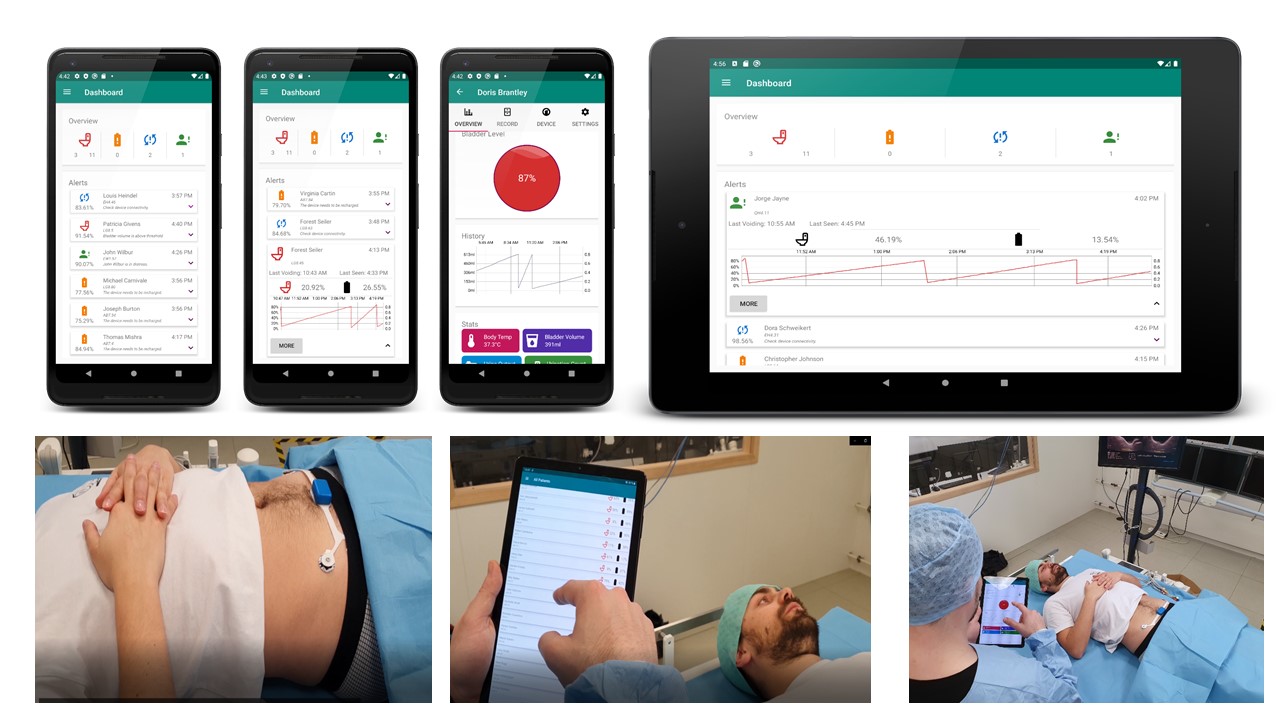
The system provides a method for non-invasive, unobtrusive, and continuous bladder monitoring in real time. It minimizes the use of indwelling or intermittent urinary catheters and the subsequent risk of infection. The sensor is held in place by strong adhesive disposable electrodes that electrically stimulate the region to measure the body bio-impedance locally. As the bladder is filled with urine, the sensor shows a change in the impedance value indicative of bladder volume change. In addition, the sensor is also interfaced with a mobile app that serves as a graphical user interface (GUI) for visualization, if needed. When the bladder is filled up to a certain threshold, an alarm is triggered on-sensor unit alerting the caregiver/health worker/UI patient. This on-sensor alarm improves the reactivity of the alert and the privacy of the user.
The emphasis is to provide accurate solutions to catalogue physiological changes in real time and provide relevant information to even non-skilled personnel. To facilitate this, a crucial feature of the sensor design includes on-board algorithms for threshold-based bladder volume assessment alerts and artifact correction. In-sensor data analytics eliminate the need for additional hardware for processing and provide artifact free information ready for interpretation by clinician or medical decision support systems. Signal processing and the artificial intelligence algorithm run into a processor integrated on a Bluetooth low energy System on Chip. The data gathered from the sensor in the absence of a highly skilled professional or complex medical systems, can be aggregated for prognosis by clinicians to monitor urological function over time.
Made for home monitoring
Patients of UI can easily use the device comfortably for home monitoring with no restrictions on movement and aid during the periodic appointments at the local clinic for urodynamic testing. Another important aspect that increases the appropriateness for continuous use is the system power design. Stringent constraints are set on the usable power, sensor accuracy, and lifetime of a wearable device due to the miniaturization and thin form factor. The sensor design is optimized for low power operation and resource sharing due to which it is able to achieve months of continuous monitoring with a single charge. The lifetime of wearable devices can be significantly improved with miniaturized energy harvesting which can be combined with low power design, intelligent even-driven detectors, energy harvesting and aggressive power management and energy efficient processing. We are also working on a second version that includes a kinetic energy harvesting to make the smart sensor self-sustained.
Additionally, the device is appropriate for environments that operate with limited staff and resources, such as health workers in critical care units of hospitals or caregivers in old age homes and disability-care clinics. This novel process, that touches the fields of mobile health, Big Data and clinical monitoring, could encourage more efficient patient management and pre-empt infection, thereby leading to a reduction of infection incidences. Such a system has not been developed before for the direct benefit of users at home, caregiving facilities, or critical care units of hospitals. This ecosystem of sensing tools, monitoring app and data logging for prognosis by clinicians will facilitate workflow, reduce human error and provide better diagnostic accuracy for the patients in the healing process. Alternatives in the form of portable handheld ultrasound devices are commercially available. These devices are bulky, need a skilled professional for analysis, suffer from low resolution imaging due to small size and cannot monitor the bladder volume continuously. The efficacy in bladder volume estimate varies across studies and each device costs between $6k-8k.
Frodo’s future adventures
We are in phase 1 of pilot studies on healthy adults with our current collaborators in Unispital, Zurich and CHUV Lausanne. The aim of the initial studies is to verify sensor performance and to establish sensitivity in measure volume change due to filling and voiding of the urinary bladder. After this, we plan to scale up to clinical trials to evaluate the sensor efficacy and efficiency in monitoring bladder volume in both clinical and at-home settings.
The most important part of the work has been done by the PhD Student Dheman Kanika, supported by other students such as Philipp Mayer and Manuel Eggimann as well as the support of undergraduate students. Frodo is currently evaluated at University Hospital Zurich with Dr. Thomas Hermanns, Dr. Denise Finke and Dr. Marko Kozumura at the Clinic for Urology along with Dr. Hugo Sax (retd.), Division of Infectious Diseases and Hospital Epidemiology, and at CHUV Lausanne with Dr Beat Roth, Department of Urology.